




Better understanding of structure and dynamics in mixtures, especially aqueous mixtures, will impact on some important scientific topics, such as green chemistry and biophysics. The focus of green chemistry is to construct commercial products and new drugs, and to preserve environment at the same time. The central part of this project deals with alcohols, alkanes, water, ethers and their mixtures. Alcohols are in general used as solvents, as fuels, in food productions and in medicine. Ethers are used as solvents and in industrial purposes.
In particular, 2-Isobutoxyethanol is used in oil industry as a non-toxic, non-expensive surfactant (Rodgers et al., 2015). Water is the most important liquid on Earth and its role in biological processes, such as protein folding, enzymatic reactions and object formation is not yet fully understood.
Electrostatic interactions play a key role in the micro-segregation of molecules and its dynamics in binary mixtures aqueous mixtures. There is interplay between the hydrophobic and the hydrophilic interactions that govern the process dynamics.
Depending on the solute chemical composition, water reorganises itself in such a way that the free energy of the system reaches a minimum, which results in the formation of domains or even micelles. The dynamics of these domains is still not completely understood, and neither is the difference between concentration fluctuations and the emerging objects in these systems.
Clearly, these two are separate phenomena, the former being present also in ideal mixtures, while the latter relates only to particular types of systems, such as the hydrogen-bonded mixtures or ionic liquid mixtures. This was confirmed in previous studies by the members of this group, both in model (Kežić-Lovrinčević, Dartois and Perera, 2015) and realistic systems (Kežić and Perera, 2012a;Kežić and Perera, 2012b)
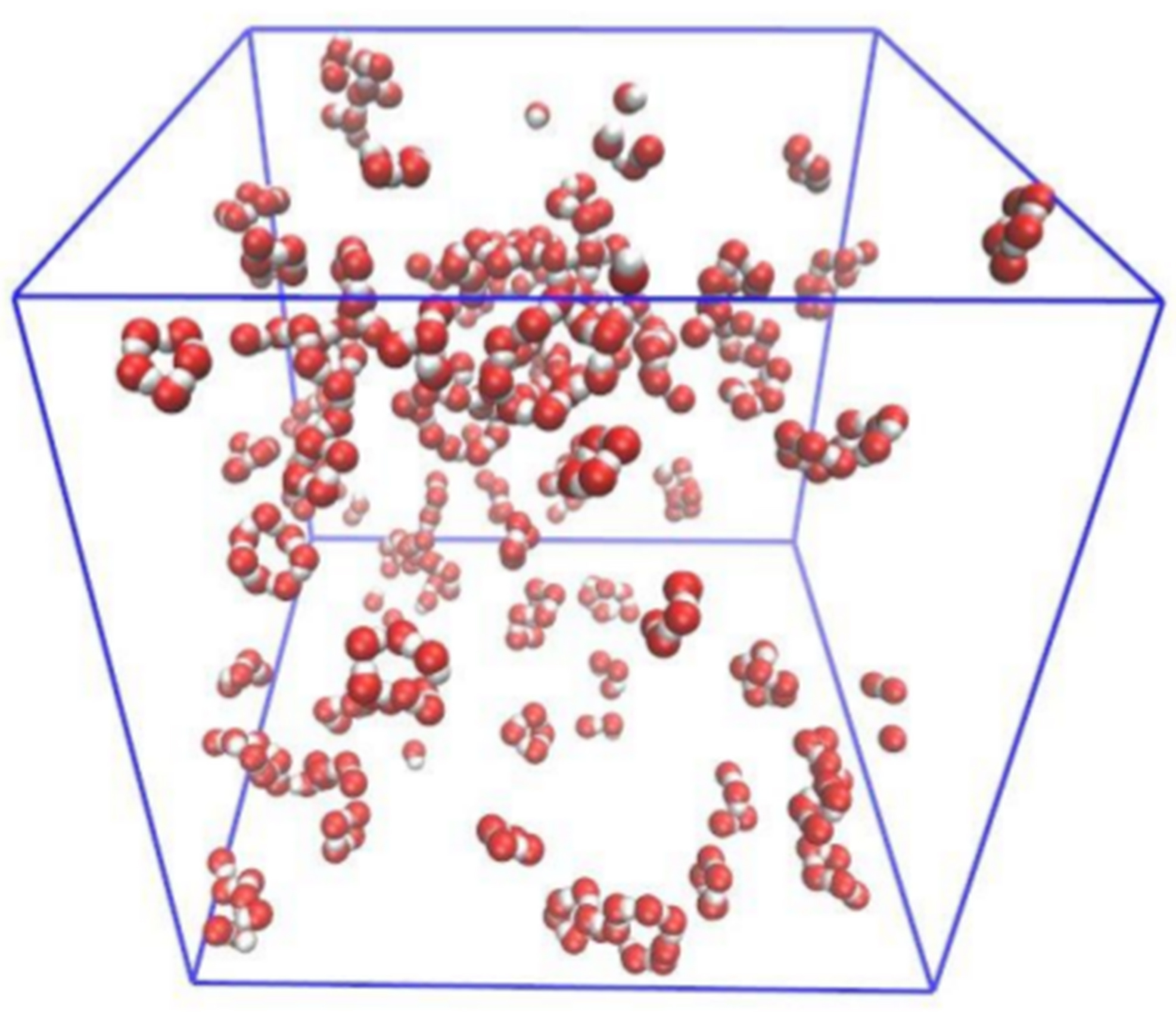
Snapshot of ethanol-hexane mixture for 20% ethanol. Only ethanol oxygen (red) and hydrogen (white) atoms are shown to visualise better the spatial organisation of ethanol molecules. M. Požar, B. Lovrinčević, L. Zoranić, T. Primorac, F. Sokolić, A. Perera, 2016.
We aim at understanding the lifetime of newly formed objects and their kinetics, which is a problem that lies at the heart of biophysics and material science. Molecular dynamics simulations and theoretical approaches ensure solutions where experimental evidence becomes questionable.
Transport properties, such as diffusion constants, viscosity and thermal conductivity, are of particular interest, since they affect most of the chemical processes. Autocorrelation functions, such as the velocity-velocity autocorrelation function give useful description about the dynamics in the liquid system. Simulations have shown to be quite useful in the calculation of the transport properties and autocorrelation functions in the recent studies of both aqueous and non-aqueous mixtures. Another great advantage of simulations is that they can enable the calculation of both static and dynamic properties of the system, giving at the same time deep insight on the microscopic level. This is particularly valuable in the study of biologically relevant systems and water mediated processes, such as the hydrophobic effect.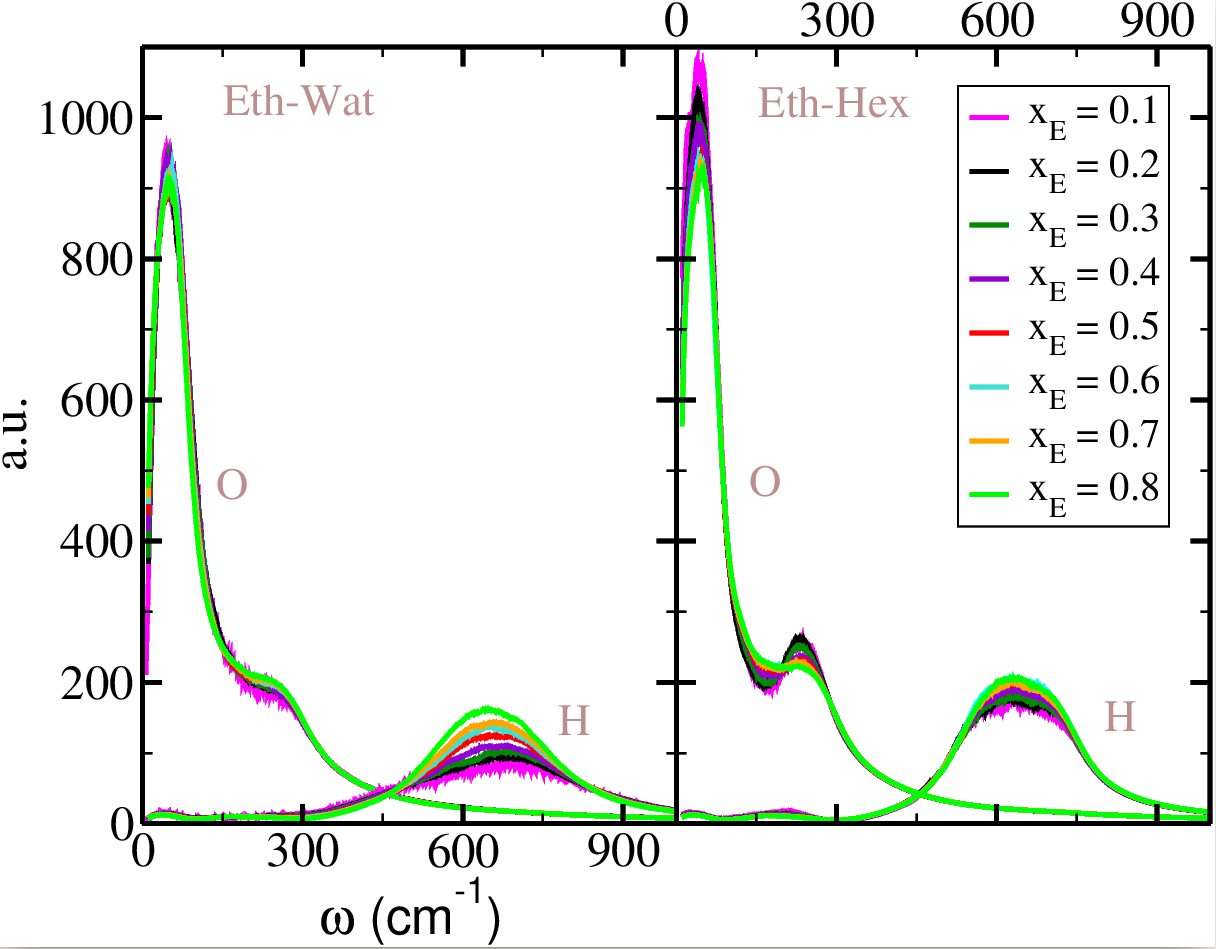
Vibrational power spectra of ethanol O and H atoms in ethanol-water (left panel) and ethanol-hexane (right panel). Color convention: xE = 0.1 (magenta), xE = 0.2 (black), xE = 0.3 (dark green), xE = 0.4 (purple), xE = 0.5 (red), xE = 0.6 (cyan), xE = 0.7 (orange) and xE = 0.8 (green). O and H labels are indicated in the panels. From: I. Jukić, M. Požar and B. Lovrinčević, Phys. Chem. Chem. Phys. 22, 23856-23868 (2020)
In the past, there have been many studies focusing on the simplest systems governed by the hydrophobic effect - aqueous mixtures. Both simulation and experimental studies have confirmed micro-segregation of the constituent molecules and the existence of water domains. But, the time evolution of these domains is not fully understood. Application of dynamic theory to realistic liquids has been mostly limited to simple liquids, typically Lennard-Jones liquids, but also weakly polar liquids.
In this perspective, it is important to understand that the extension of these approaches to associating liquids does not consist simply of accounting for yet another type of interaction - the associating interactions, but it would rather consist of taking into account the new physical phenomena that result from this interaction, namely micro-segregation. In order to better appreciate this point, it is noteworthy to recall that the hydrogen bonding of water under cooling undergoes a dramatic change in the local clustering. Obviously, this exceptional plasticity of water needs to be either described as a consequence of the type of interactions (hydrogen bonding), or to be directly incorporated into the theory.




Publications
Conferences
• M. Požar and B. Lovrinčević participated at the CECAM workshop "New frontiers in liquid matter" July 4, 2022. - July 7, 2022. They presented their work with posters: "Local entropy/energy fluctuations in molecular liquids: the role of the site-site bridge function" (Lovrinčević) and "Clustering versus fluctuations in monohydroxy alcohols" (Požar). CECAM program and list of participants can be found here.
• M. Požar and B. Lovrinčević participated at the CECAM workshop & meeting "Recent progress in the statistical mechanics of solutions through Kirkwood-Buff integrals and related approaches", September 20-22, 2021. B. Lovrinčević gave a talk entitled "Do the Kirkwood-Buff Integrals represent a signature of the microscopic state of mixtures?" and M. Požar presented a poster "Why aqueous piperidine has a scattering pre-peak and pyridine not? Insights from Kirkwood-Buff integrals,scattering data and computer simulations". CECAM program and list of participants can be found here.
• I. Jukić, M. Požar and B. Lovrinčević participated in the online 11th Liquid Matter Conference, July 19-23, 2021. The group work was presented during the poster session.
• I. Jukić participated in the online Journée Systèmes & Matière Complexes conference, November 16, 2020. where he gave a talk "Diffusion and memory: simple versus complex liquids".
• B. Lovrinčević and M. Požar participated in the EMLG 2019 conference in Kutná Hora, Czech Republic, September 8-13, 2019. The group work was presented in two talks: “The role of many-body correlations in liquids: simple disorder versus complex disorder” by B. Lovrinčević and “Microscopic origin of the scattering pre-peak in aqueous propylamine mixtures: X-ray and neutron experiments versus simulations” by M. Požar.
• M. Požar participated in the 4th Grandmaster Early-Career Workshop in Phyisics, held at the University of Split, Faculty of Science, Split Croatia, September 1-7, 2019. with the poster presentation: "Microscopic origin of the scattering pre-peak in aqueous propylamine mixtures".
News
• M. Požar recieved an award from our Faculty of Science as the most productive scientist at the Department of Physics on 25. 05. 2022.
• B. Lovrinčević and M. Požar participated at the Center of Excellence of the Split-Dalmatia County with lectures on 12. 02. 2022. Details about their lectures can be found here
• M. Požar gave an online invited presentation for a project "Ms. or Mrs.? Dr." of the Croatian Society of Chemical Engineers (CSCE) on 08. 03. 2021.
• Our group gave an invited online presentation about group project activities for the Physical Society Split meeting on 13. 01. 2021.
• M. Požar participated at the Center of Excellence of the Split-Dalmatia County with two lectures about spectroscopy on 24. 10. 2020. and 07. 11. 2020. Center of Excellence of the Split-Dalmatia County works with gifted students from elementary and high schools in developing their skills and knowledge in the STEM fields. About these events
• Our group was visited by prof. Katsura Nishiyama, a researcher from Meijo University in Nagoya, Japan on 01.03.2020. Prof. Nishiyama gave a talk entitled: "Application of nanoscale photoenergy-conversion materials prepared in liquid phase: from biomarkers to afterglow systems".
• Aurelien Perera, a researcher from Sorbonne University in Paris and a long-time group collaborator, has given a seminar and a workshop for students at the Faculty of Science, University of Split on 14.01.2020. Students had an opportunity to learn about the basics in statistical mechanics and molecular dynamics simulations. About these events (in Croatian!)
University of Split
Faculty of Science
Ruđera Boškovića 33
21 000 Split, HR
+38521 619245
bernarda@pmfst.hr